Achievemint Studies
While at Evidation Health, I worked as a solo designer on a new product for running clinical studies. To date, Achievemint Studies has produced peer-reviewed learnings published in major medical journals.
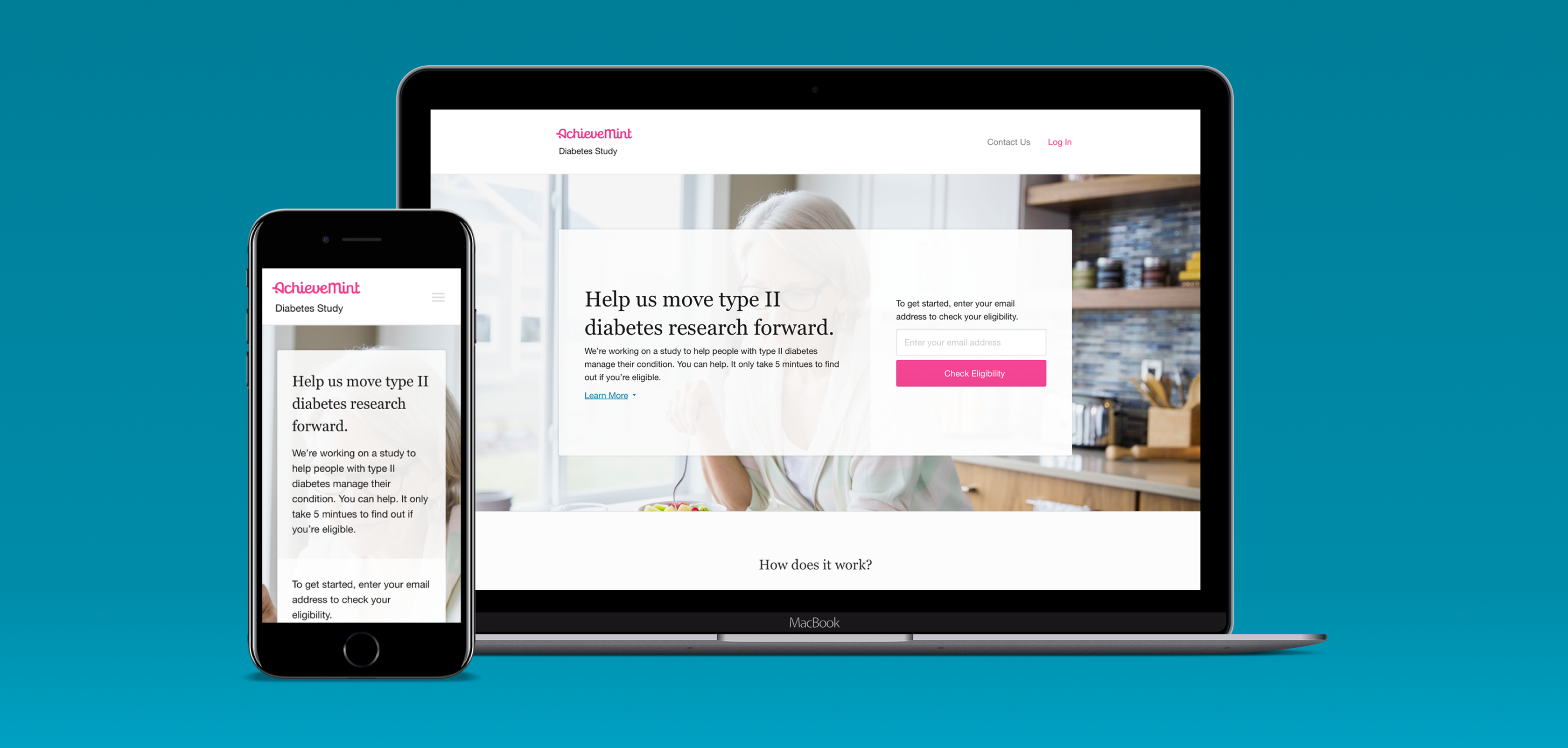
Shortly after the founding of Evidation Health, there was a need for a solution to be created to help fulfill the company's main mission at the time — to prove the efficacy of digital health solutions.
To accomplish this, we needed to run clinical studies where we could observe how digital apps and devices affected peoples health. These studies could have been run the old-fashioned way, by recruiting participants in-person at clinics or hospitals and requiring them to check in multiple times over the course of the study. However, we saw an opportunity to greatly improve this process for all parties involved - better results for our clients, an enjoyable experience for study participants, and a better way to run clinical studies at scale.
Research
Being completely unfamiliar with what clinical studies were and how clinical studies were run, my first step was research. It was crucial that I understood who and what I was designing for.
During conversations with the internal health outcomes research team who design studies, I discovered that most studies follow a roughly standard setup for screening and enrolling participants. However, after participants are enrolled, different studies divert wildly. They might ask their participants to do anything from fill out a survey to go and complete a task in their day-to-day life. Examples of different studies and their different expectations for participants.
Challenges
Given what I learned during research, the biggest challenge was designing a product that was flexible enough to accommodate the wide variety of different needs studies have.
An additional challenge, in line with the flexibility of the study platform, was the difference between study participants. A study may require participants who have a specific disease and those who have that disease tend to be older. Or the subject of a study may be something as simple as how a specific app or device affects people’s steps, which requires a broader range of different participants.
Both of these challenges helped guide my decision making when designing the product. If a specific feature or functionality made the product less flexible or made it more difficult for specific audiences to use, it needed to be revisited.
Enrolling in a Study
Enrollment in a study asks participants the right questions to determine if they are eligible to participate. This is critical to study's success as if a study doesn't recruit enough eligible participants, it fails.
The questions asked during enrollment range from the age of a participant to when they were last treated for a specific disease. It's important that the product can adapt to asking those questions. It's even more important for participants to understand and be able to quickly answer them.
I designed a system that displayed one question at a time, while always showing the overall progress through enrollment. This allowed participants to quickly answer questions while understanding how long enrollment was going to take them. It also was flexible enough to accommodate any number of questions or for longer, more complex questions to be asked of participants during enrollment. An example of a question that may be asked of participants during enrollment.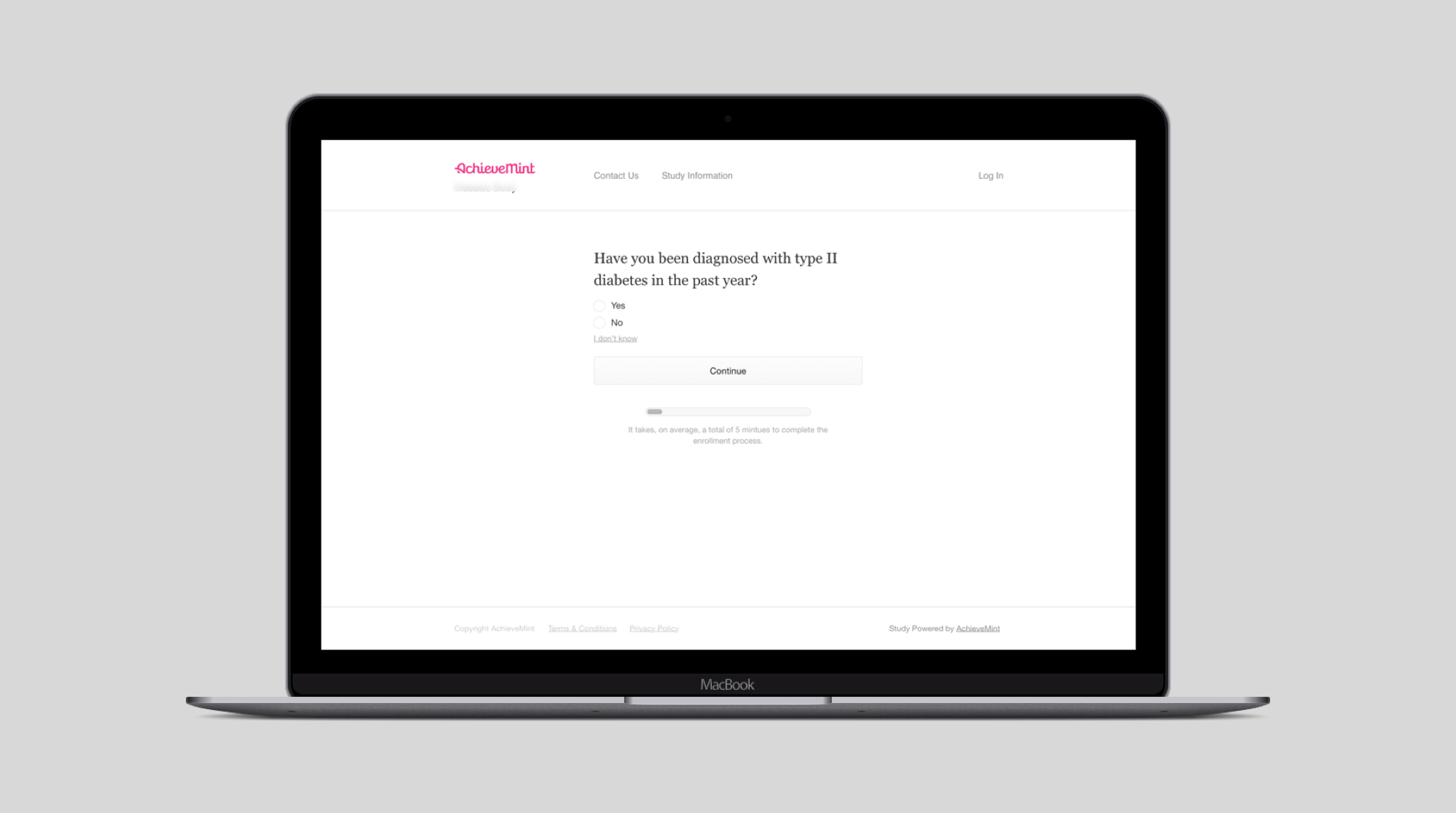
Participating in a Study
During a study, a participant is asked to complete a number of contributions (or tasks). If what they need to do at any given time isn't clear to them, they might not complete that contribution. If participants can't complete their contributions and provide the necessary data, a study can't succeed. This made a well designed participation experience absolutely critical.
The solution I designed separated each contribution out into its own element, all of them sharing the same visual structure. This allowed a different mix of contributions to be used for each study depending on the needs of that study. In addition, the shared visual structure allowed new contributions to be added easily as Achievement Studies grew.
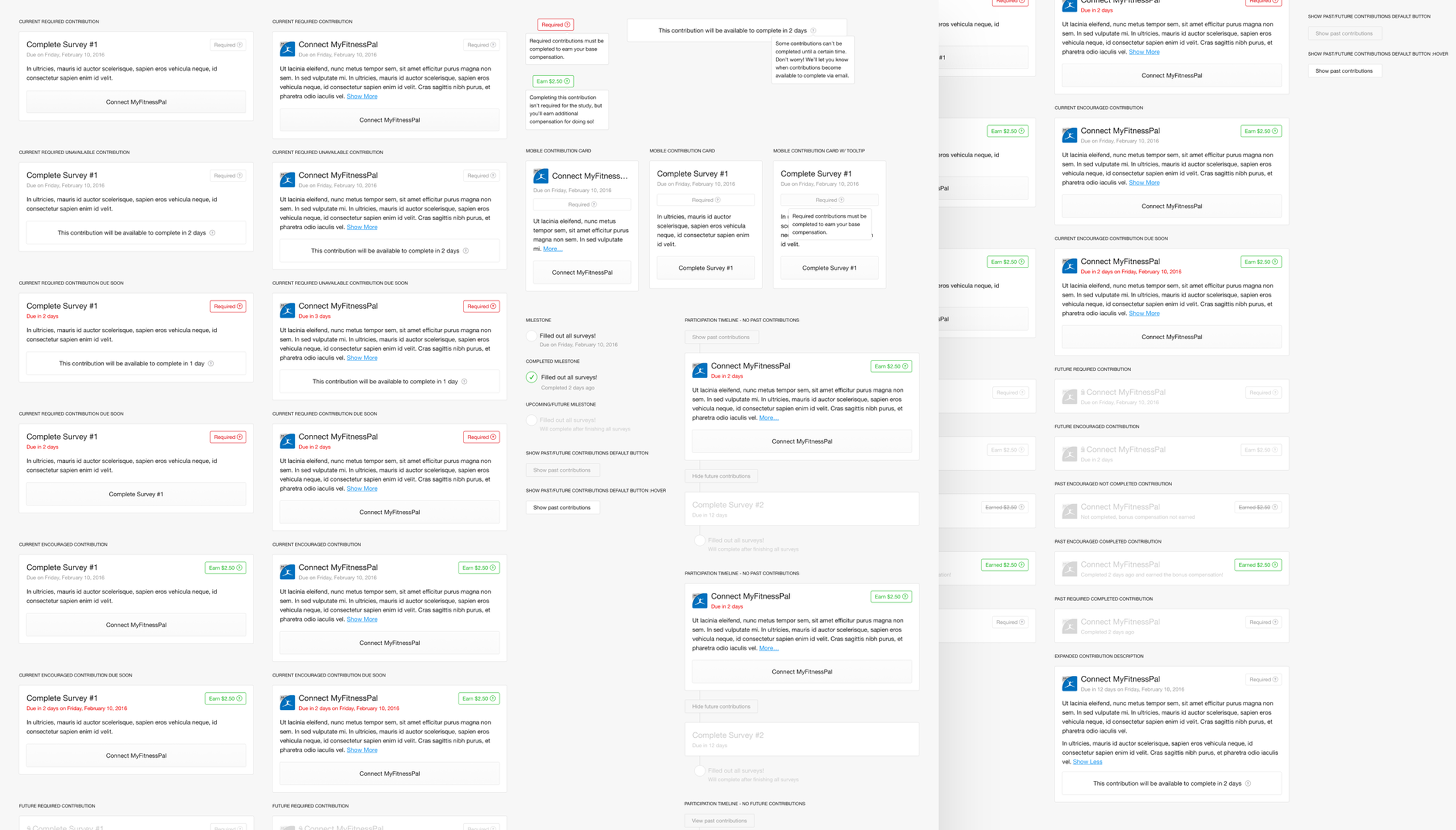
To give a participant a sense of their progress throughout a study, contributions were aligned on a timeline. Participants were able to view this timeline in its entirety, but only be able to act on the contribution that currently needed their attention. This made certain that a participant understood everything that they would be required to do during a study, but kept them focused on completing the task at hand. The shots here show the explorations and iterations in how to best show contributions to members during their participation. The various states and types of contributions that can be in a study.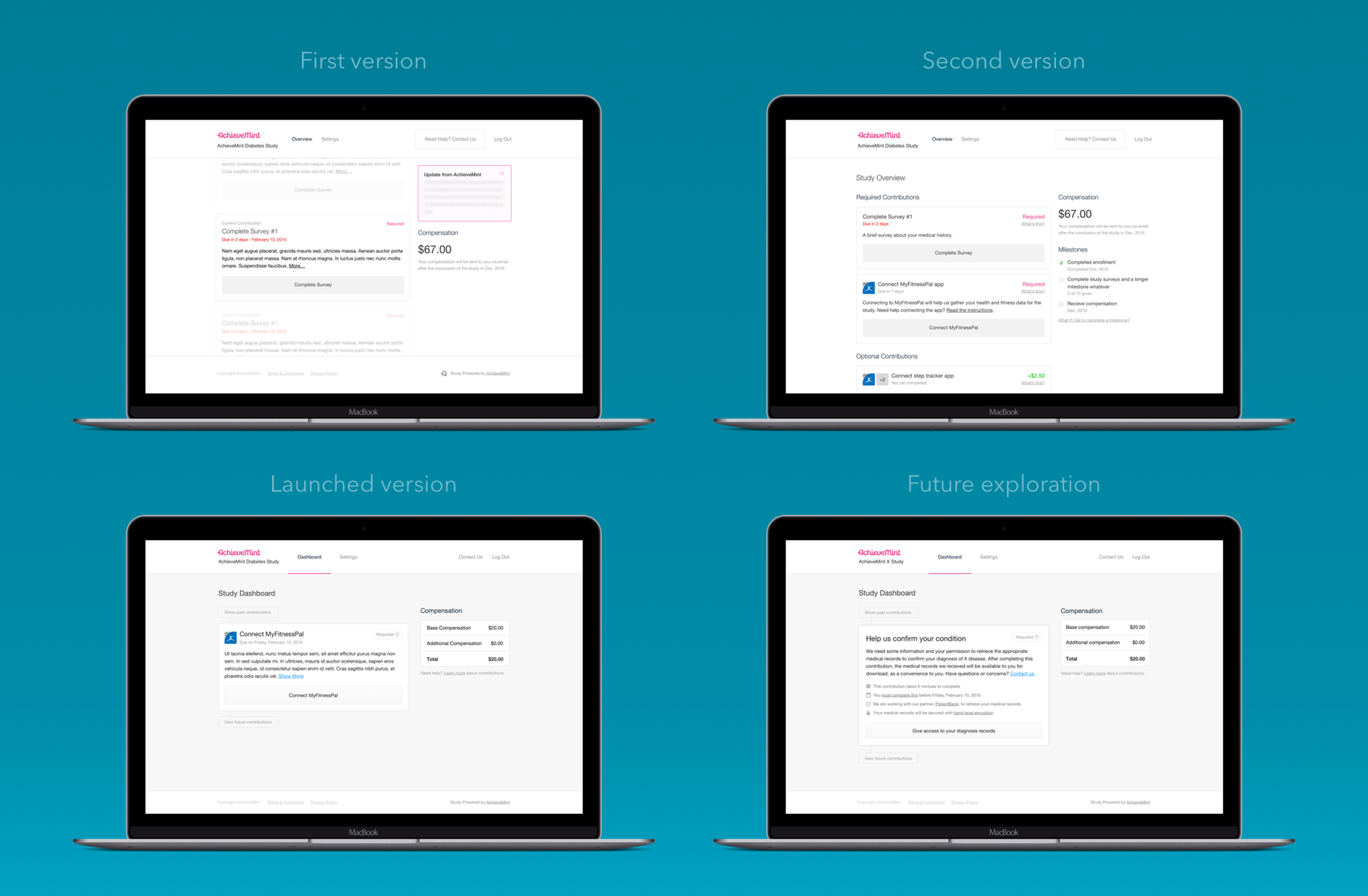
What I Learned
The flexibility of the product was one of its strongest elements. It was able to adapt to a wide variety of different needs and use cases. In multiple studies, additional functionality needed to be added. Achievemint Studies has been able to accommodate those new needs with little additional work needed.
While flexibility has been a success in Achievemint Studies, it has been a struggle to design enrollment that adheres to the strict standards required of clinical studies. There's still a lot of work to be done in improving enrollment to make it even easier for participants to understand their involvement in the study and increase enrollment rates.